Motiva® x Meghan Trainor
GRAMMY Award-winning hitmaker Meghan Trainor received breast augmentation surgery with Motiva SmoothSilk Ergonomix® Implants. For Meghan, self-confidence and body positivity have always been essential. After significant weight loss and becoming a mother of two, Meghan faced a challenge many women can relate to — feeling confident in her own body again.


bla
“I’ve thought about breast augmentation for years and I’m glad that Motiva Implants® became available just as I was ready to make a change. I chose Motiva SmoothSilk Ergonomix® because of the unique design that adapts to your body as it changes position,1,2,3 the SmoothSilk® surface which is scientifically shown to lower inflammation4 and their outstanding safety profile5 with less than 1% device related complications5,6. I am so happy – I have the natural look and feel I want without compromise.”
Meghan Trainor, Motiva SmoothSilk Ergonomix® Mini 290cc
Meghan's Motiva®Journey
Meghan opens up on her personal journey — sharing what inspired her and why she chose Motiva Implants®. Hear how Meghan drew her inspiration, how she felt shortly after the procedure and how she's moving in her new Motiva Implants®.
-
Meghan's Motiva® Journey
Meghan Trainor’s journey is a reminder of why we do what we do. At Motiva®, we’re Motivated by You—designing implants that support your confidence, your choices, and your lifestyle.
-
Meghan's Post-Surgery Update
We love that Motiva Implants® could offer Meghan the options, safety,5 and results she was looking for in this new chapter.
-
Meghan's Results with Motiva®
Confidence looks good on Meghan Trainor and we couldn’t be happier for her choice to get Motiva Implants®.
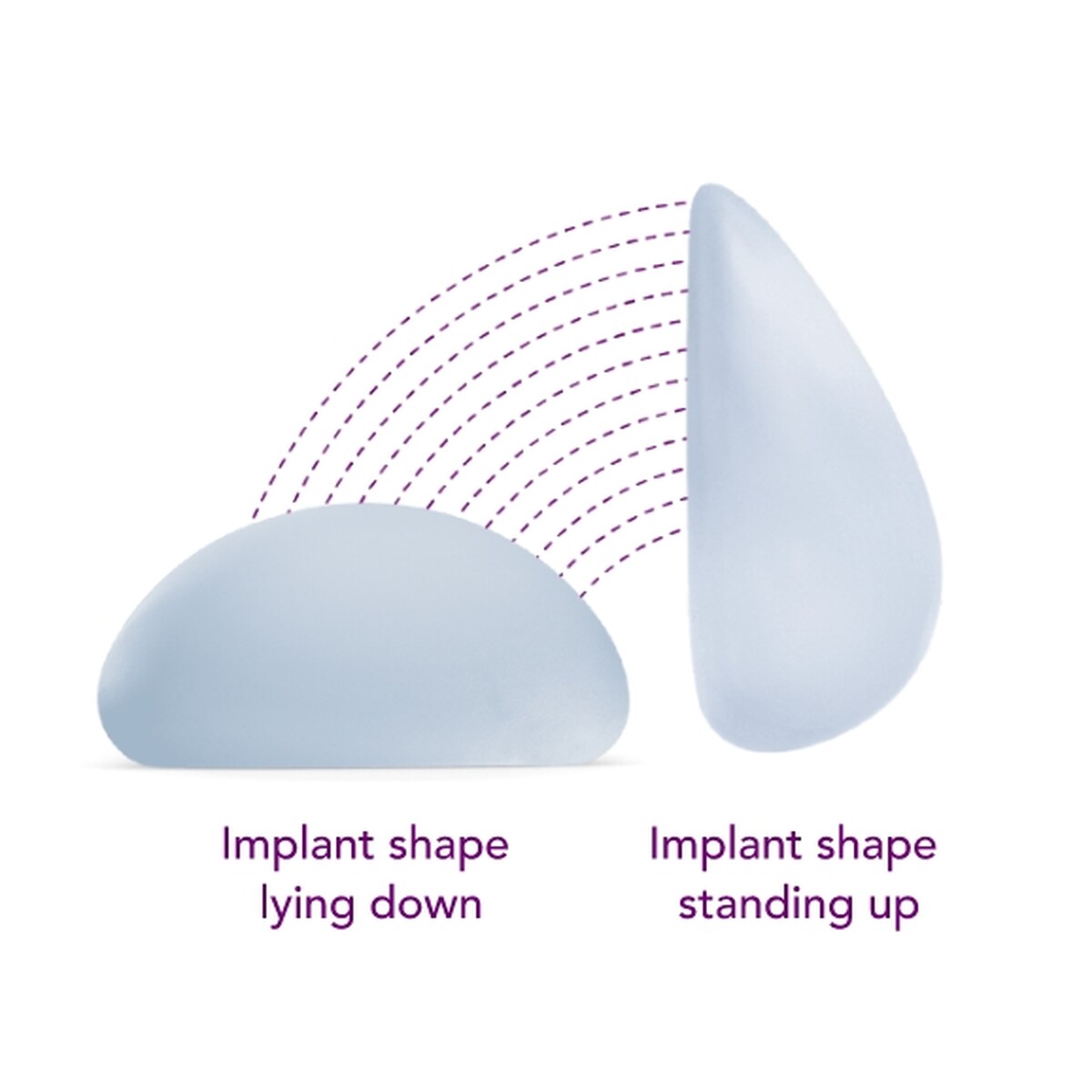
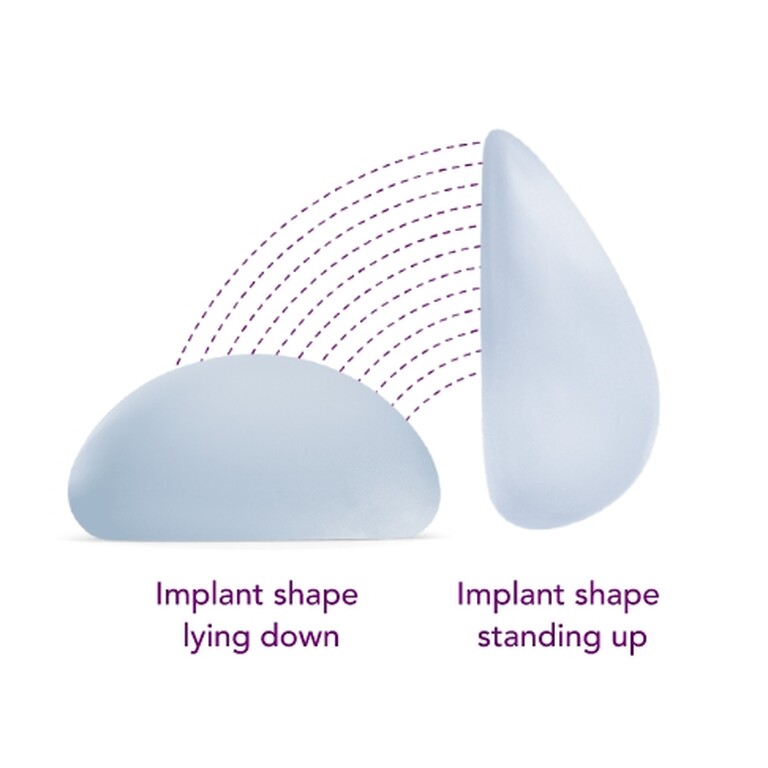
Meghan chose Motiva SmoothSilk Ergonomix®
Ergonomix® implants are unique to the implant market, as they can adapt shape as your body changes positions, showcasing a round shape when lying down and a teardrop shape when standing up.1,2,3 This breakthrough implant is designed to be inserted through a small incision6 and minimize scarring.
Benefits of SmoothSilk®
-
Soft breasts.1,5
-
Low capsular contracture rates1,5 (when the scar tissue surrounding the implant tightens or hardens).
-

No reported primary device-related incidence of BIA-ALCL.6
The buzz has been electriccccc!
Meghan’s story struck a powerful chord with fans and publications alike. As Seen In...

Motiva® Influencer Special Offer Surgery Window (3/6-5/31) is closed.
Participants who claimed their special offer code and had surgery between 3/6-5/31 have until 6/30 to redeem their code. Please refer to the email with special offer code and follow instructions to redeem.
Motiva® Influencer Special Offer FAQs
1. How long does the offer last?
Patients must complete their breast augmentation surgery with Motiva Implants® between 3/6/25 and 5/31/25 (surgery window) and redeem their code between 3/6/25 and 6/30/25 (redemption window) in order to receive their $250 gift card.
2. Why don’t I see the special offer code field on MotivaImagine®?
If you don’t see the special offer code field when either completing your implant registration or accepting your registration entered by your surgeon, it likely means based on your surgery, you are not eligible for the special offer. Per the offer terms and conditions, patients who have had a breast reconstruction are not eligible for the offer. Patients who have only received 1 Motiva® implant are also not eligible for the offer. Please confirm your surgery date is between 3/6/25 and 5/31/25 and your redemption date is between 3/6/25 and 6/30/25. If you believe you should be eligible for the offer, please email marketing@motivausa.com for assistance.
3. Why isn’t my code working when I try to enter it on MotivaImagine®?
Please ensure you registered for your MotivaImagine® account using the same email address you submitted on the original special offer landing page. If you attempt to use a different email, your special offer code will be recognized as invalid. You must claim your special offer code prior to your surgery date. If your special offer code is claimed after your surgery date, you would be considered not eligible for the program and your submission will be denied.
If you’ve previously claimed a code through the special offer program, you will be unable to make another claim. The offer is open to legal residents of the United States at least 22 years old at the time of their Qualifying Surgery who have received their Motiva Implants® in any of the fifty (50) states, the District of Columbia, or Puerto Rico (“Participant”). Licensed health care professionals, employees of licensed health care professionals and Motiva employees are excluded from participation and are not eligible to receive benefits from the Program.
4. Why didn’t I receive my gift card, even though I successfully submitted my code on MotivaImagine®?
Please allow 3-4 days for the team to review your submission and issue your gift card. If you still haven’t received your gift card after this period of time, please email marketing@motivausa.com for assistance. Please note, you must claim your special offer code prior to your surgery date. If special offer code is claimed after surgery date, you would be considered not eligible for the program and your submission will be denied.
References
1. Aitzetmuller-Kleitz ML, Yang S, Wiebinghaus P, Wellenbrock S, Ozturk M, Kuckelhaus M et al. Complication rates after breast surgery with the Motiva Smooth SilkSurface® silicone gel implants – A systematic review and meta-analysis. Clin. Med. 2023, 12,1881. doi:10.3390/jcm12051881
2. Establishment Labs®, TR-001038: Rheological analysis of silicone filling gels of Motiva Implants® and other brands’ silicone filling gels using the BTC-2000. Data on File.
3. Establishment Labs®, DDD-002: Device Description Document for Sterile Silicone Breast Implants Motiva Implants® Ergonomix® Round SmoothSilk®/SilkSurface®. Data on File.
4. Doloff JC, Veiseh O, de Mezerville R, et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nature Biomedical Engineering. 2021. doi:10.1038/s41551-021-00739-4
5. Establishment Labs® Holdings Corp. Establishment Labs® Notes Presentation of 5-Year Results from Motiva® U.S. IDE Study at the Aesthetics MEET 2025. https://investors.establishmentlabs.com/press-releases/press-releases-details/2025/Establishment-Labs-Notes-Presentation-of-5-Year-Results-from-Motiva-U-S--IDE-Study-at-The-Aesthetic-MEET-2025/default.aspx. Published March 20, 2025. Accessed March 20, 2025.
6. Establishment Labs®, Post-Market Surveillance Preliminary Results Q4 2024. Internal Data on File.
7. Zeplin PH. Narbensparende Brustvergrößerung: Erfahrungen mit über 500 Implantaten. Handchirurgie · Mikrochirurgie · Plastische Chirurgie. 2021;53(02):144-148. doi:10.1055/a-1307-3917
Patient giving testimonial is compensated by Motiva®. Testimonial given represents the real-life experience of the patient.
Important Safety Information
The sale and distribution of this device is restricted to users and/or user facilities that provide information to patients about the risks and benefits of this device in the form and manner specified in the approved labeling provided by Establishment Labs® and Motiva® USA. Federal (USA) Law restricts this device to sale by or on the order of a physician.
The Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants are indicated for breast augmentation for women at least 22 years old. Breast augmentation includes primary breast surgery to increase the breast size, as well as revision surgery to correct or improve the result of an original primary breast augmentation surgery (i.e., revision-augmentation). Breast Implant surgery is contraindicated in women with active infection anywhere in their bodies, with existing cancer or pre-cancer of their breast who have not received sufficient treatment for those conditions, or who are currently pregnant or nursing. Adequate studies have not been performed to confirm the safety of breast implant surgery in women with these conditions or under these circumstances; therefore, if you have any of the conditions or circumstances listed above, breast augmentation surgery with implants should not be performed at this time. Failure to take into consideration these contraindications may increase the risks involved with breast implant surgery and have the potential to cause harm. Patients should be advised that key complications have historically been associated with silicone gel breast surgery and implantation of silicone gel breast implants including, but not limited to, capsular contracture, implant removal, reoperation, infection, and rupture. Further, breast implants are not lifetime devices and patients should visit their healthcare professional, as recommended. For more detailed information about the benefits and risks of Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants, please visit: www.motivausa.com or call Motiva at 1-800-924-5072.