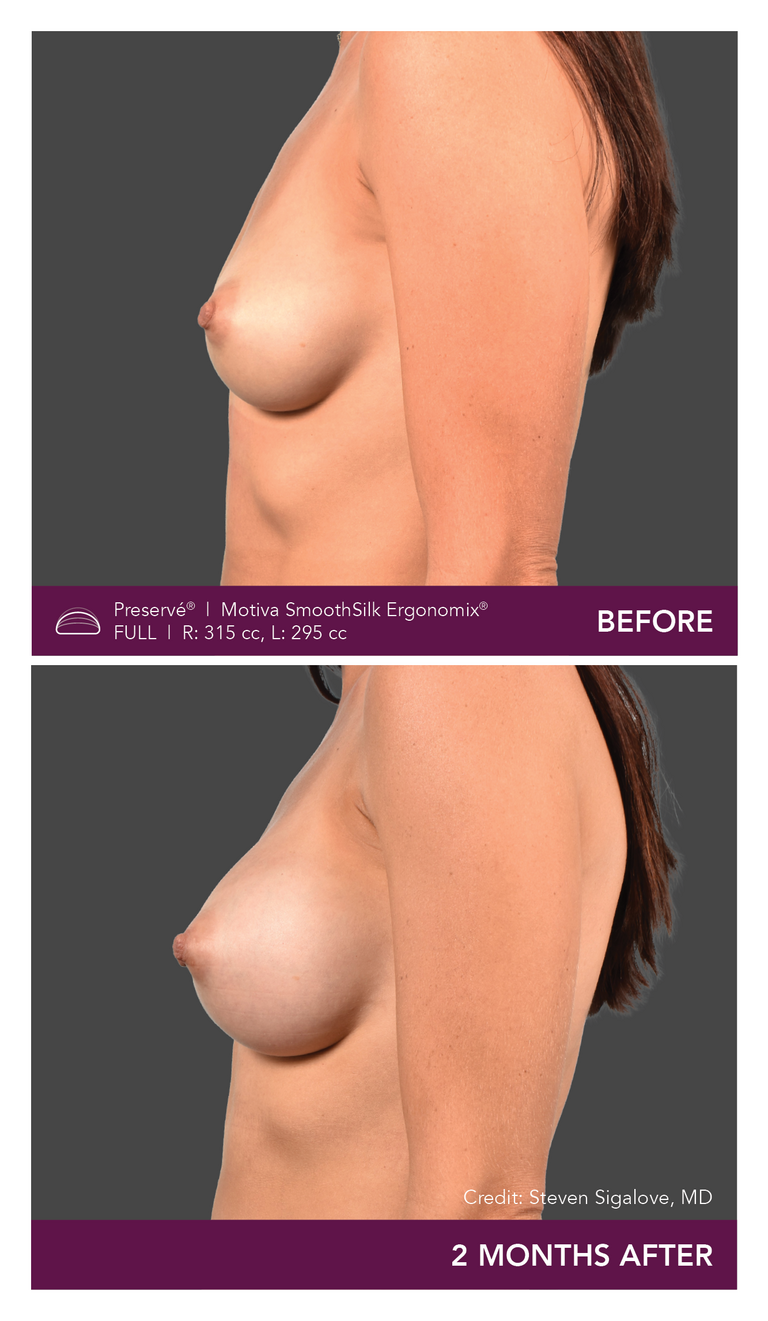
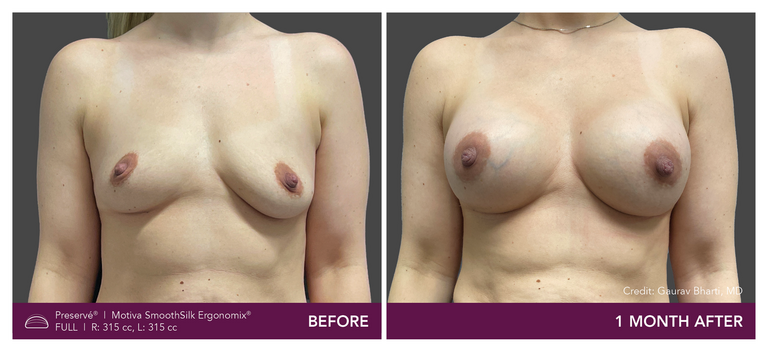
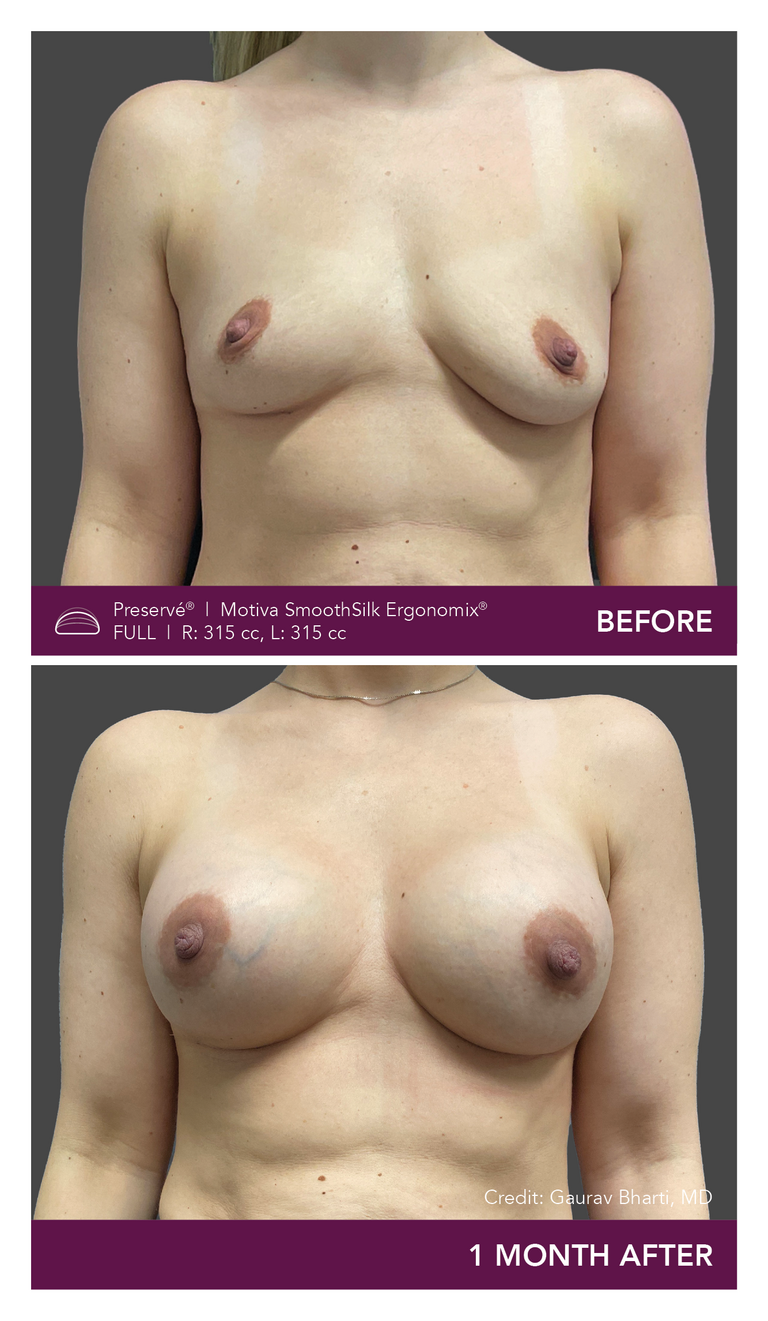
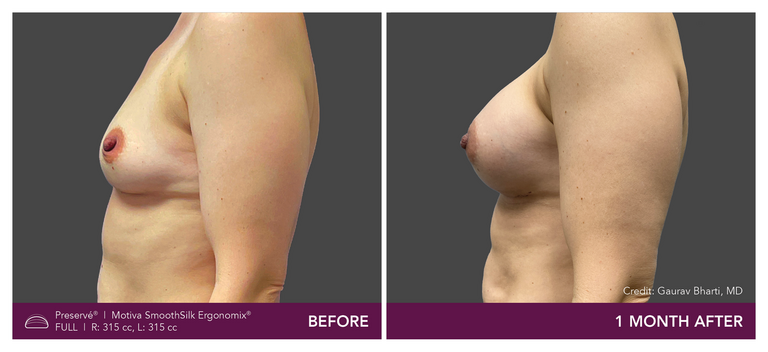
.
Preserve your natural breast tissue
Preservé® is an advanced, less invasive* breast enhancement technique made possible by
patented technologies and designed specifically to preserve your natural breast
tissue, nipple sensation and chest muscles.1,2
*Compared to traditional breast augmentation.
Preservé® may be right for you if you are considering:
-

A breast augmentation for the first time (primary breast augmentation)
-

A breast augmentation, with a lift, for the first time (primary breast augmentation mastopexy)
The art of Preservé®
The Preservé® procedure is designed to be performed through a less invasive* technique
with minimal anesthesia3, allowing you to have a fast post-procedure recovery.1,2


Step 1 • Your surgeon will take specific measurements of your breast that will allow them to perform a less invasive technique and maximize your native breast tissue.3*This approach allows your surgeon to preserve the inner structures of the breast and use an implant tailored to your unique anatomy, designed to achieve your desired breast volume.


Step 2 • After numbing the area, your surgeon will create a small incision in the inframammary fold and use the Motiva® Channel Separator to create a tunnel9 without cutting the tissue. This provides access to the space where your breast implant will live.


Step 3 • Next, your surgeon will insert the Motiva® Inflatable Balloon to gradually push your tissue structures aside, creating a precise pocket that matches the size of your breast implant.11,12


Step 4 • Finally, your implant will be inserted in the space that was created, preserving your natural breast tissue functionality, including nipple sensation and your chest muscles.1,2
*As compared to traditional breast augmentation
Preserving Your Natural Breast Tissue
Watch the video below.
Preservé your
-

Breast Anatomy
Preservé® offers a less invasive* breast augmentation designed to preserve your natural breast tissue functionality, including nipple sensation and your chest muscles.1,2
-

Breast Stability
Preservé® is designed to maintain the inner support structures of the breast, providing enhanced implant stability with 0% inferior malposition (lowering of the implant placement) as shown through a clinical study at three years.1,2
-

Peace of Mind
When you choose Motiva Smoothsilk Ergonomix® implants with Preservé®, you can benefit from the low inflammatory response8 and low complication rates13 of the Ergonomix® implants and the innovative Preservé® technique that allows for smaller incisions7 and the ability to conserve your native breast tissues for a quick recovery1,2. The best of both worlds.
*As compared to traditional breast augmentation
Featuring our
Motiva SmoothSilk Ergonomix®
Breast Implant
Motiva SmoothSilk Ergonomix® implants are unique to the implant market, as they can adapt shape as your body changes positions, showcasing a round shape when lying down and a teardrop shape when standing up.4-6 This allows the implant to realistically mimic the look, feel and movement of a natural breast.4-6
This breakthrough implant is designed to be inserted through a small incision7 making it a perfect choice for Preservé® procedures.


What women are saying
“I would always look at myself and feel like my breasts didn’t quite match my body. They feel so natural, they look natural, and honestly, I don’t even remember how they looked before. And the recovery, for me, was the best part because I honestly never felt like I had surgery. You might hesitate at first out of fear, but once you’ve done it, you’re so happy, and you think, “Why didn’t I do this sooner? ”
Fernanda
Preservé® Patient
Ergonomix® Demi 180cc


Testimonial given reflect the real-life experience of patients who have undergone implantation of Motiva Implants* with the Preservé® procedure. Results may vary and we do not claim, nor should it be assumed, that any individual experience recounted is typical or representative of what any other individual may experience. These testimonials are freely and voluntarily provided, but patient may have received benefits such as free product in exchange for their participation in Establishment Labs training programs. Please speak with your doctor or local healthcare provider to determine if the Preservé® procedure and Motiva Implants® are right for you.
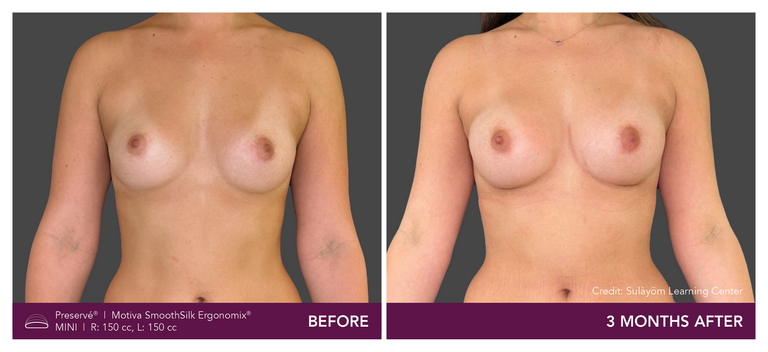
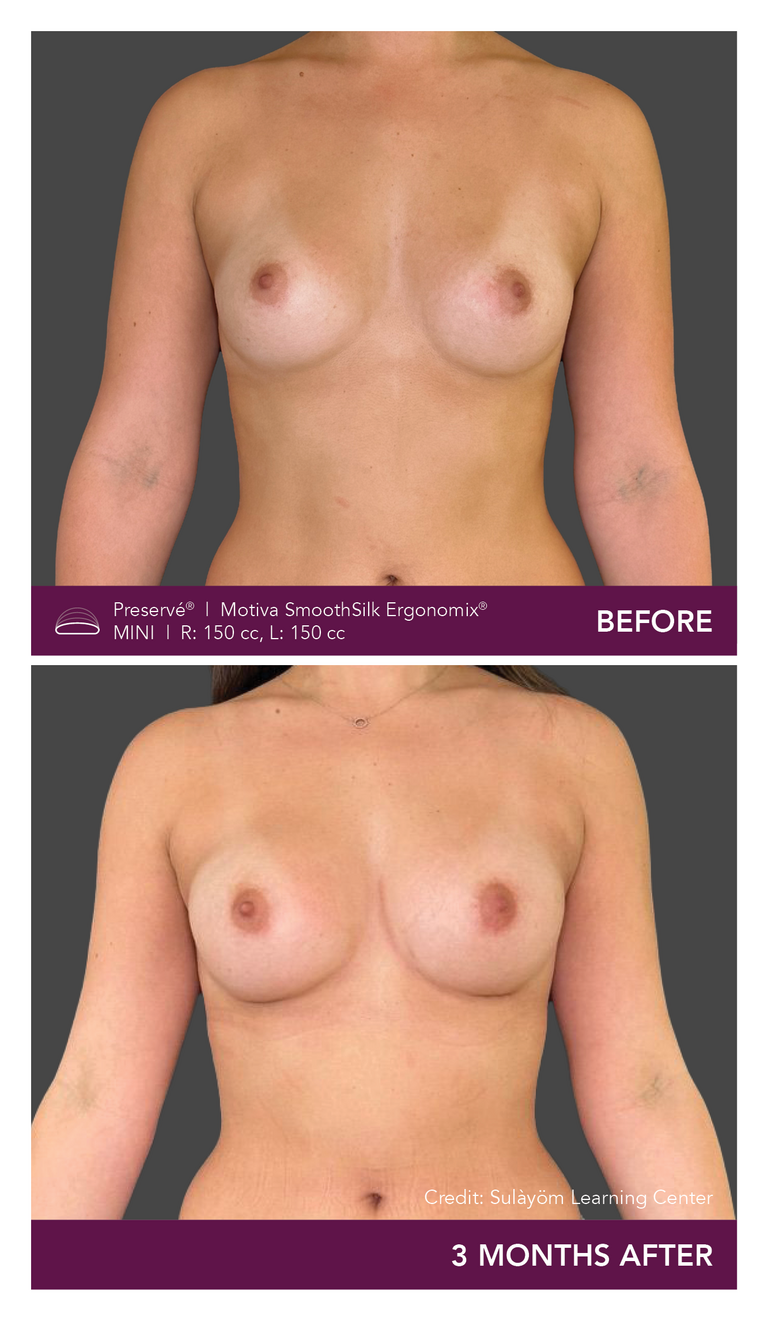
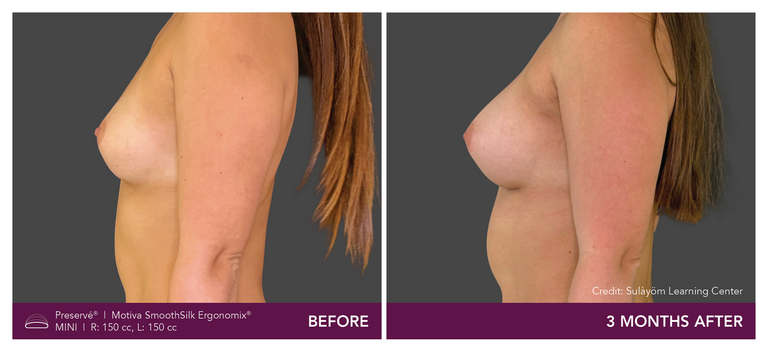
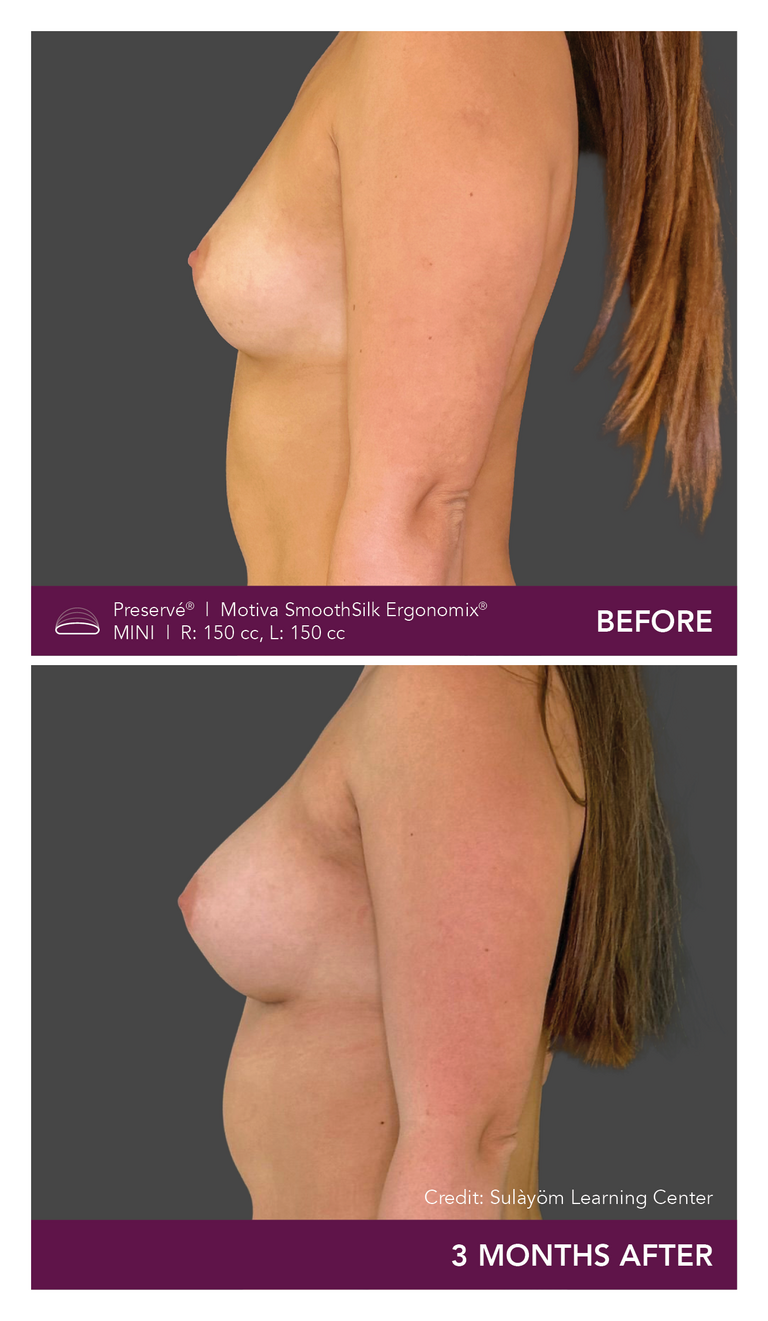
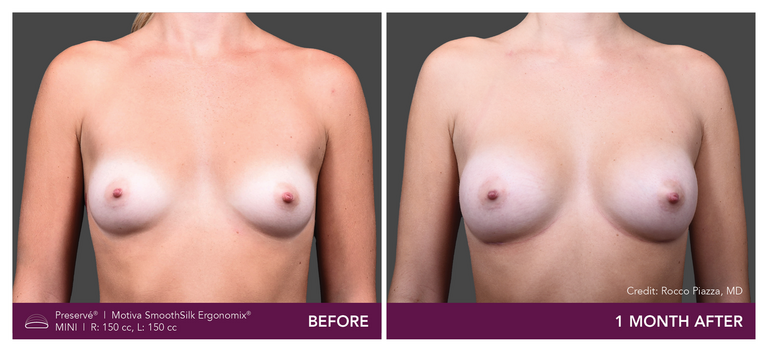
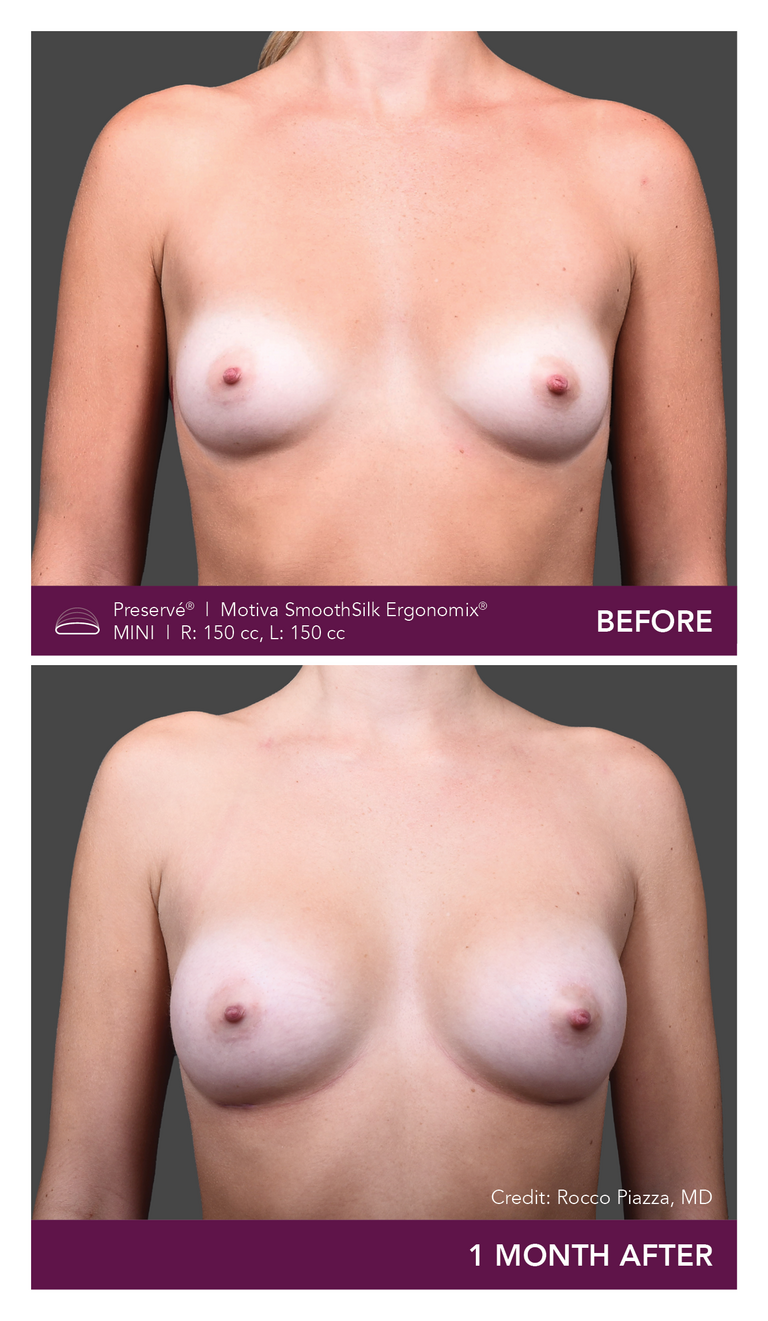
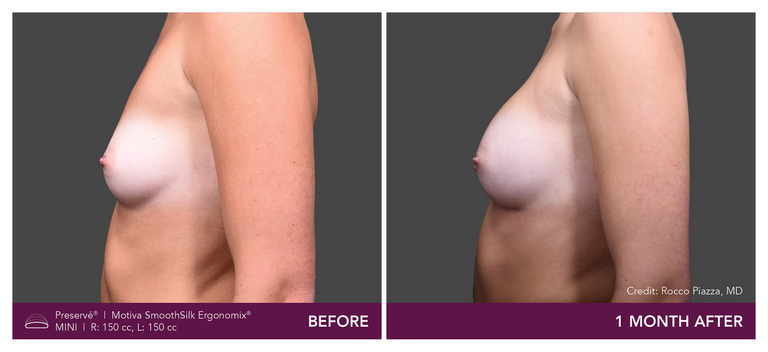
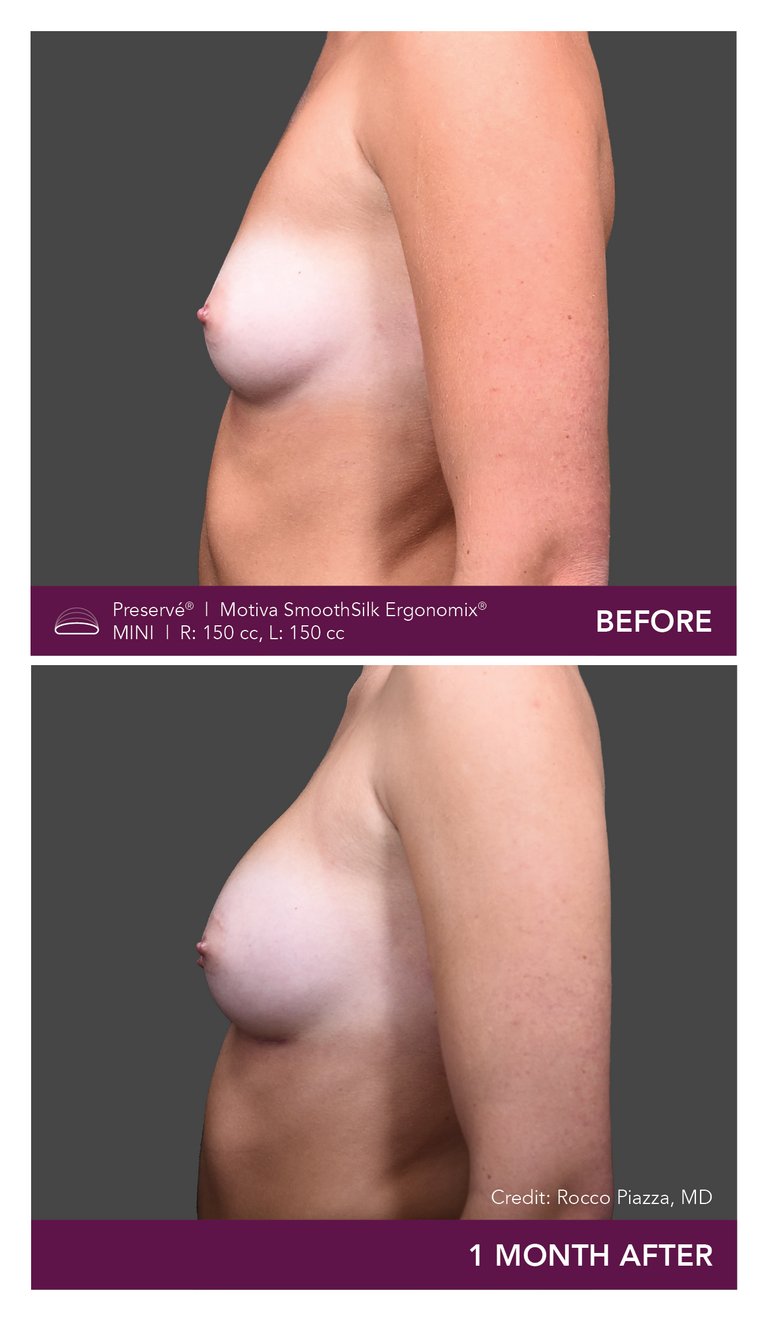
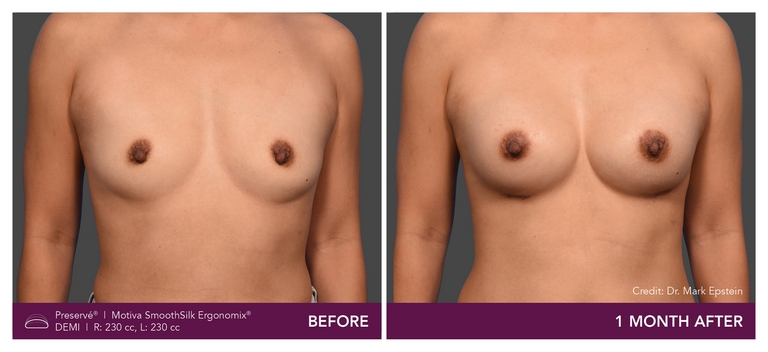
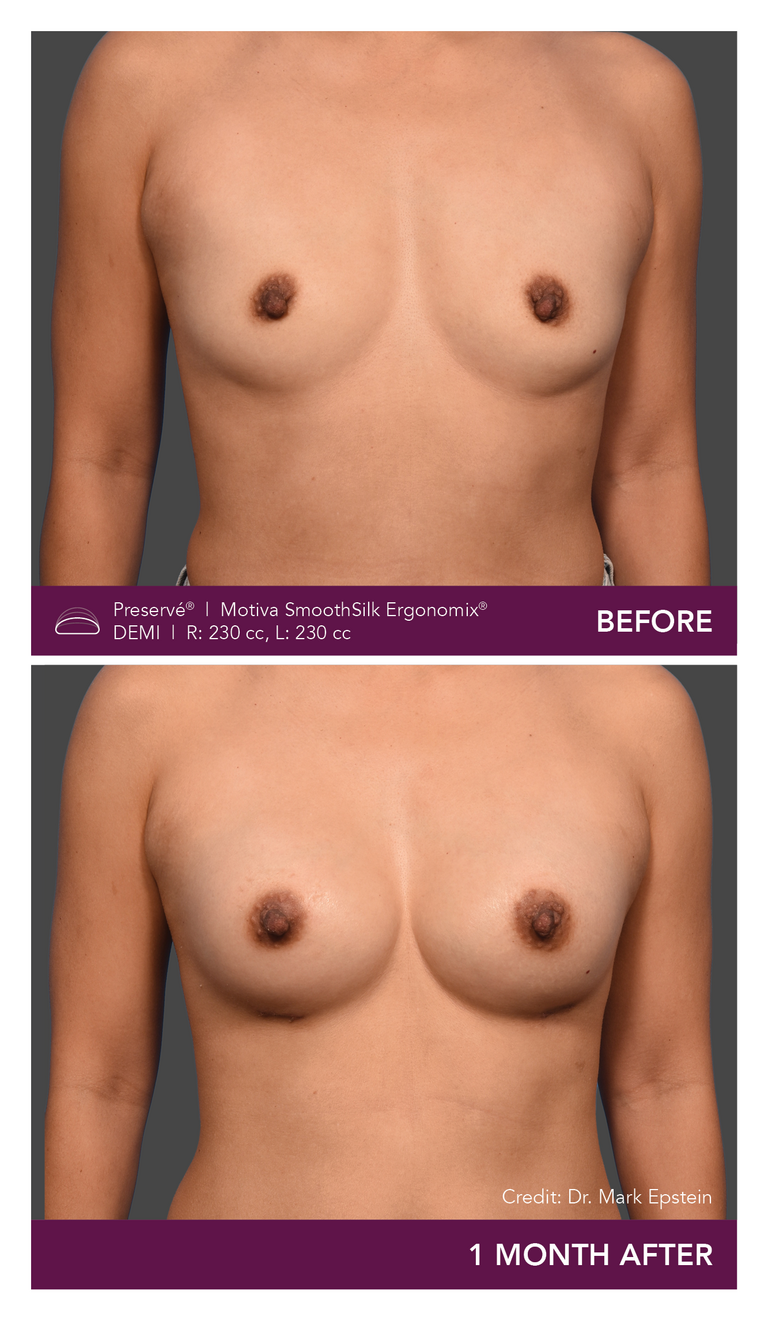
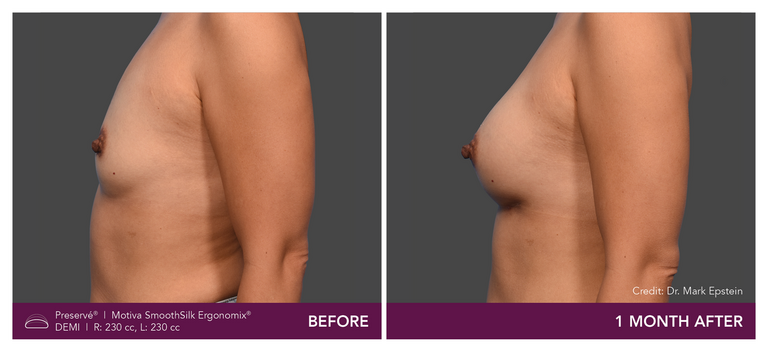
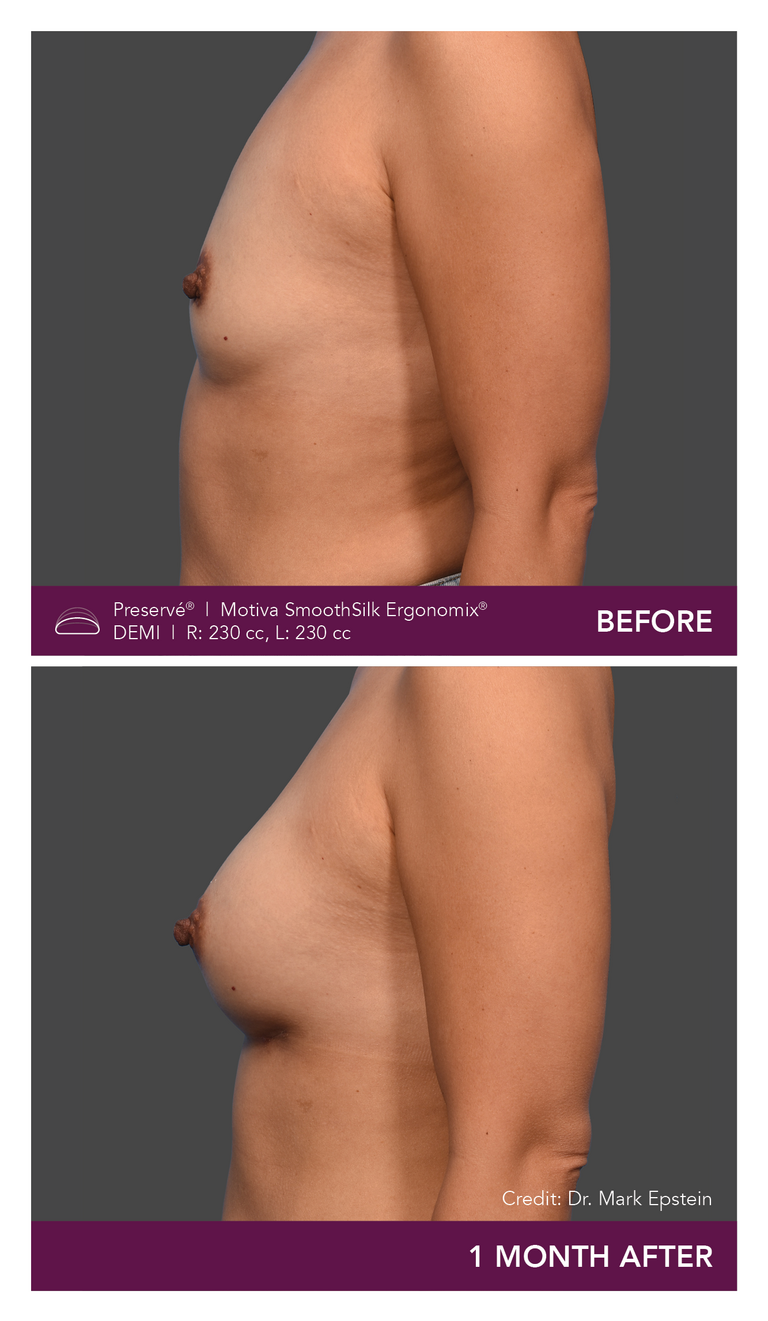
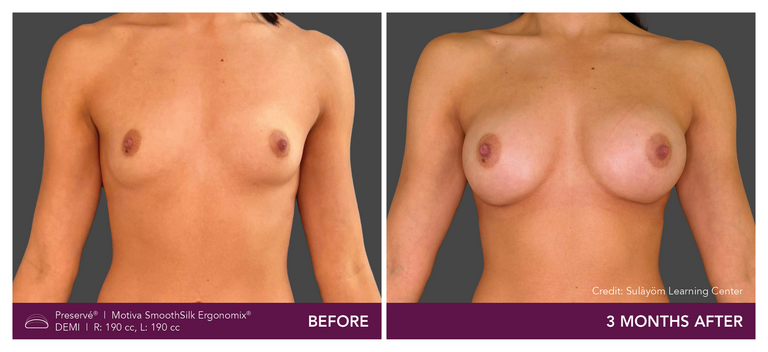
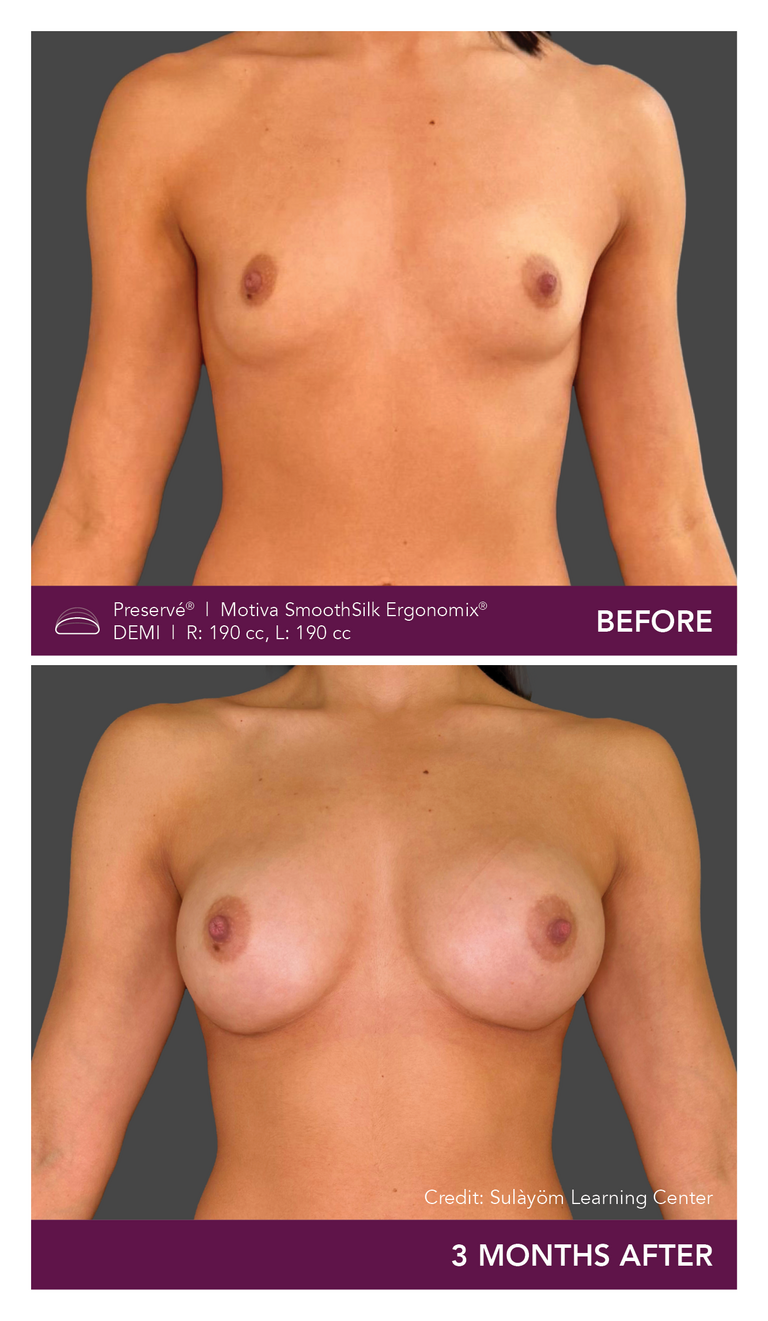
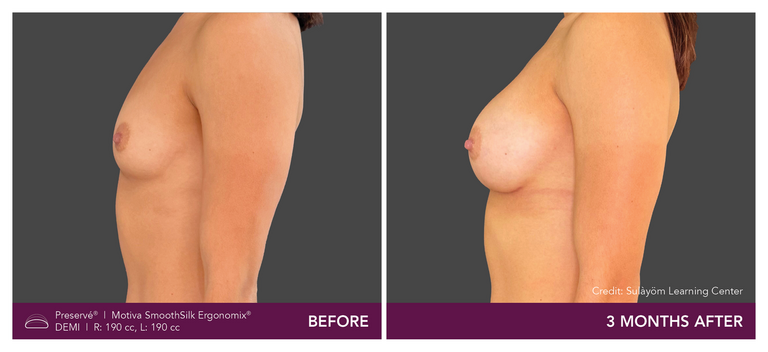
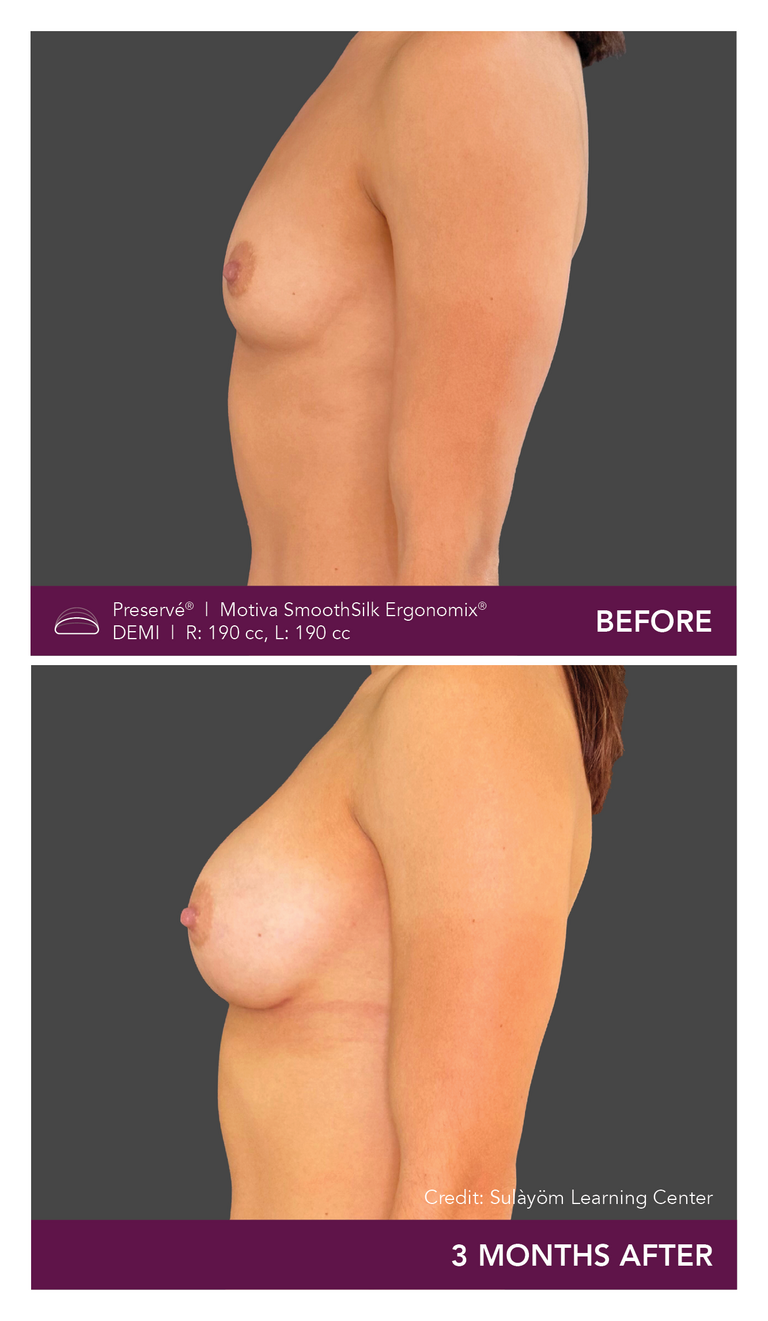
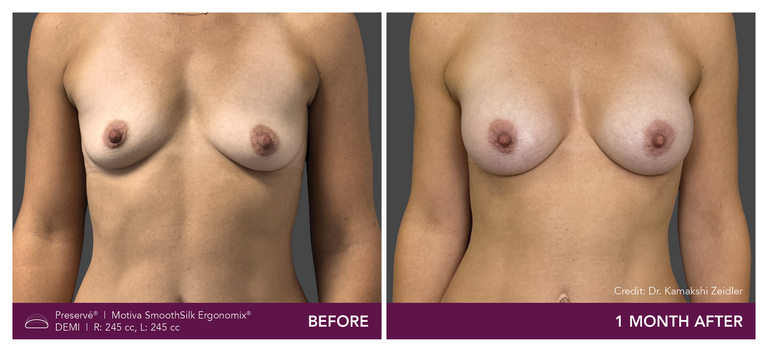
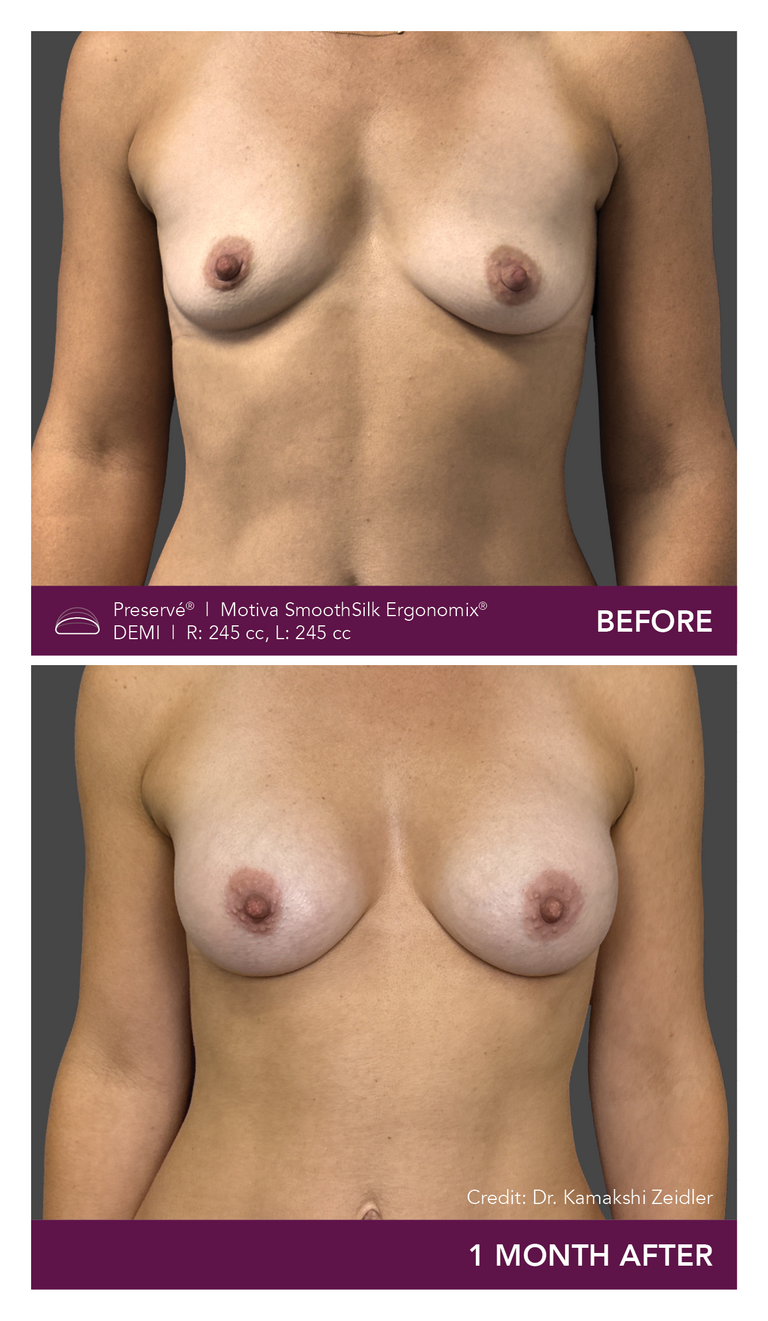
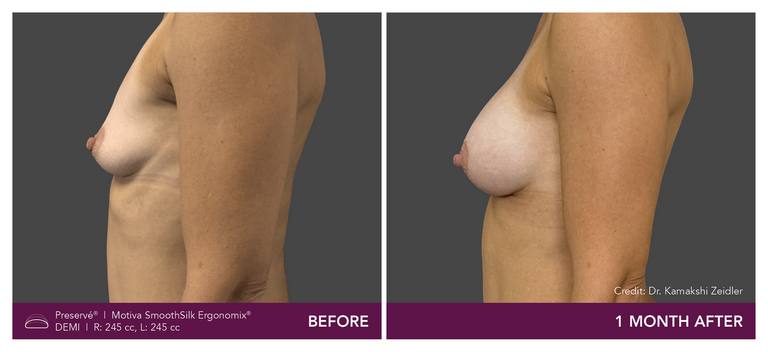
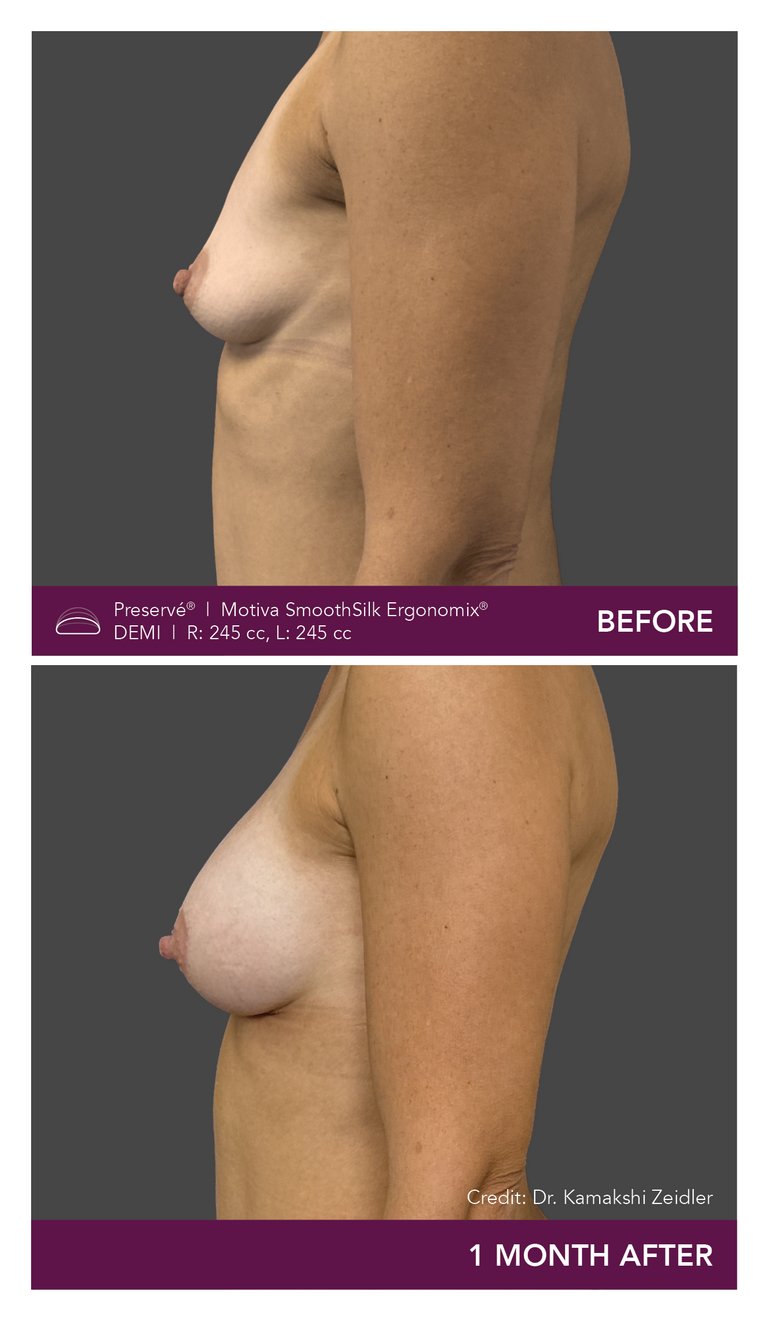
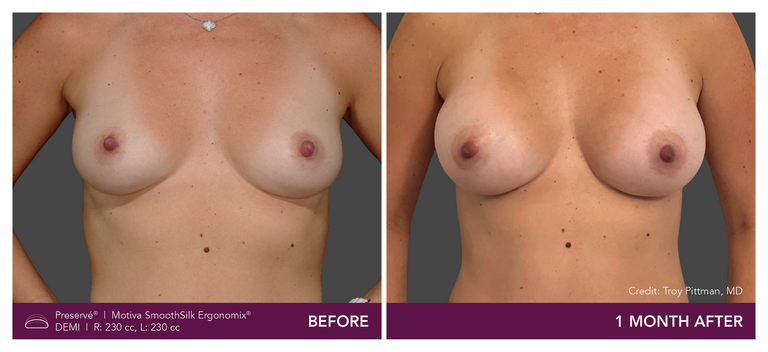
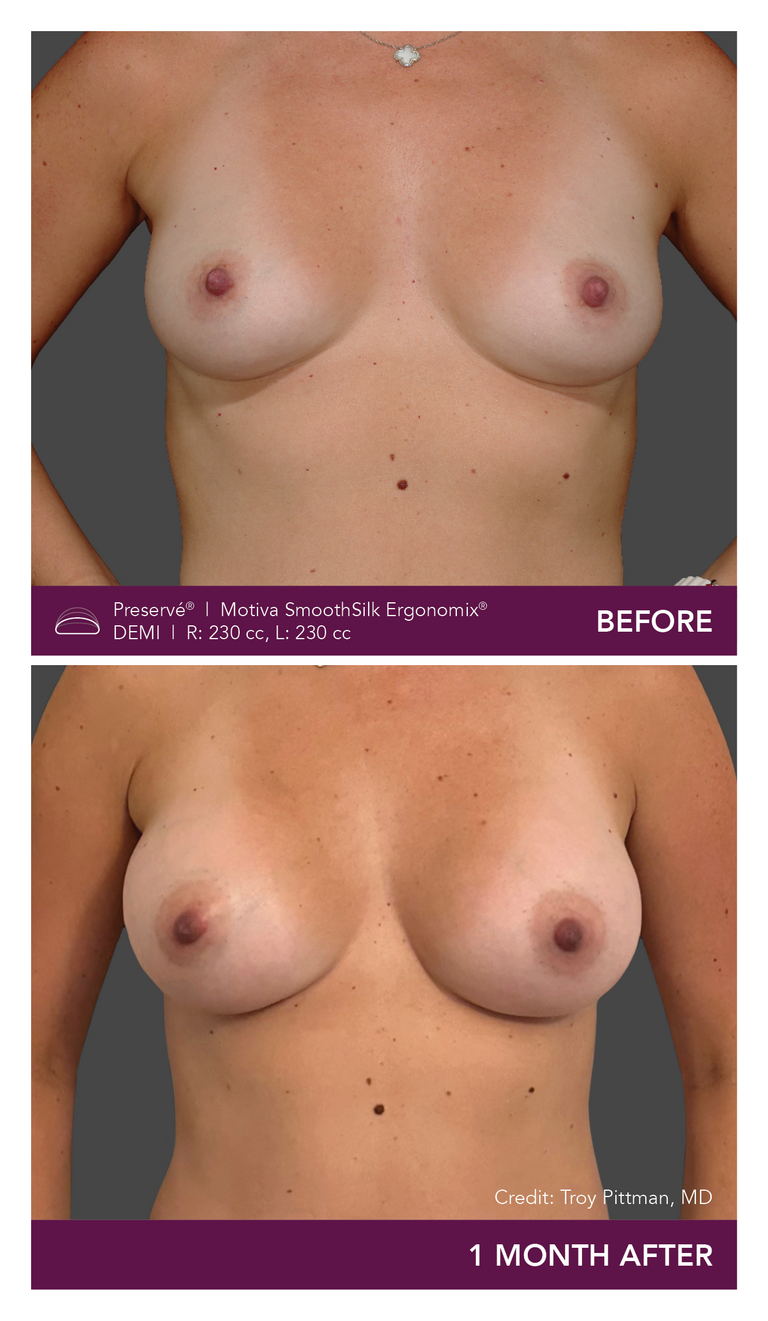
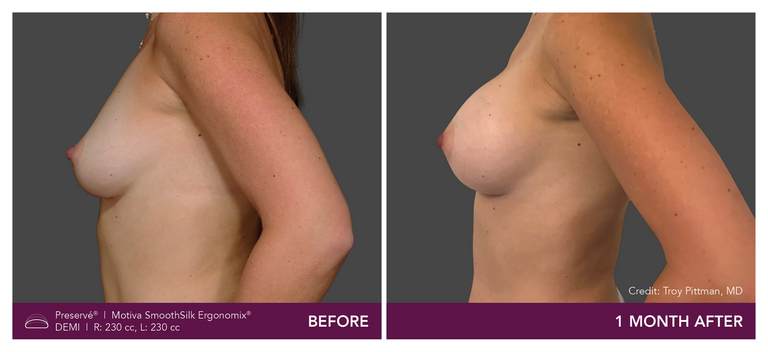
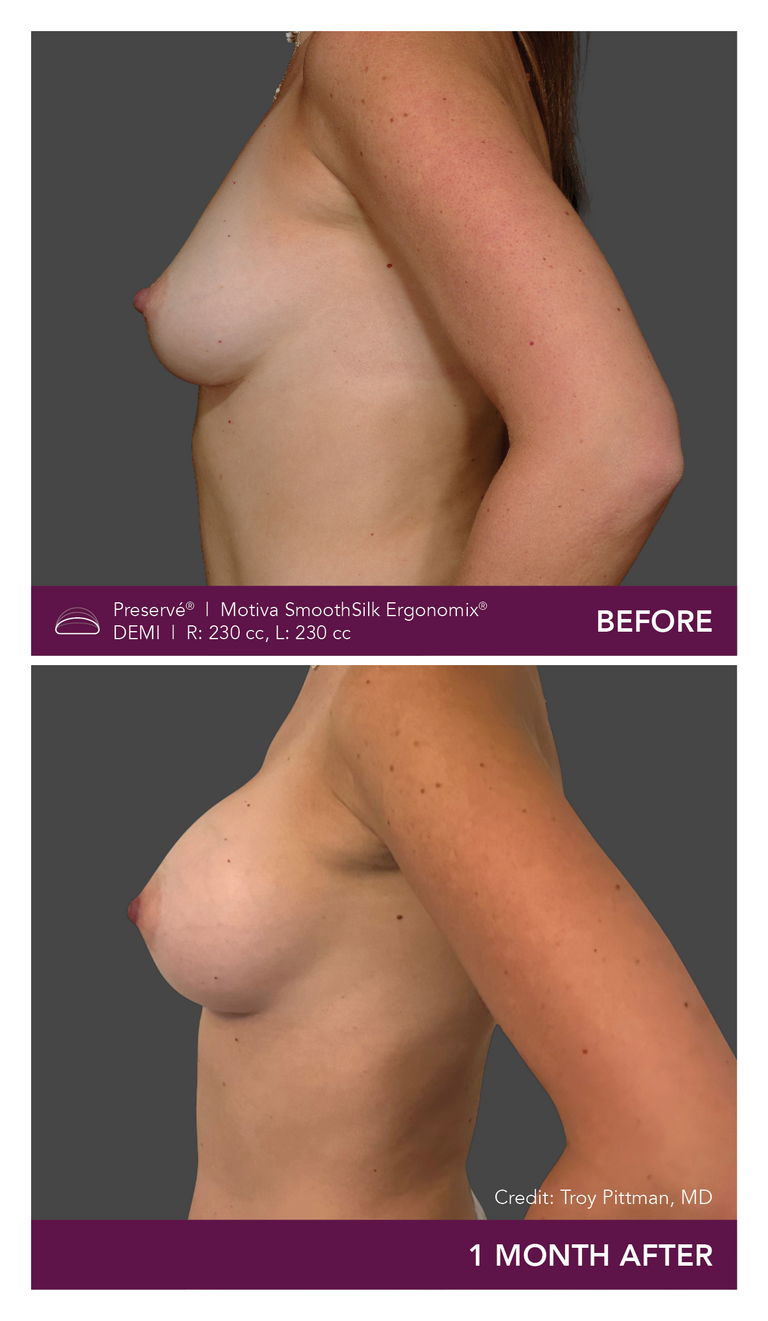
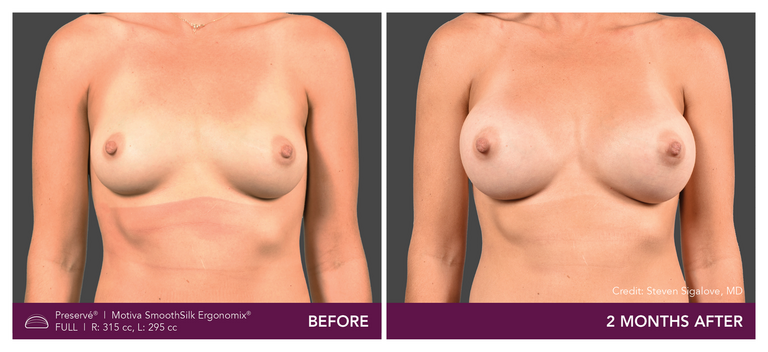
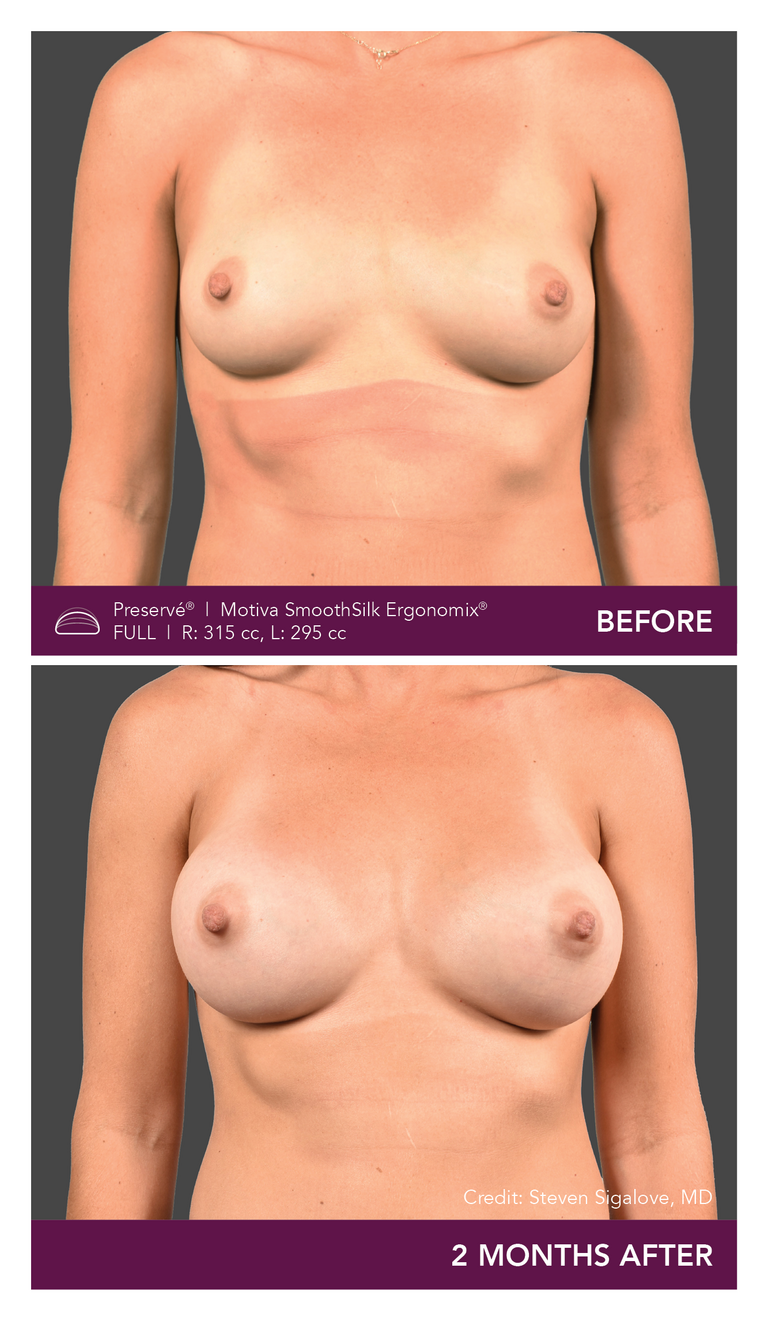
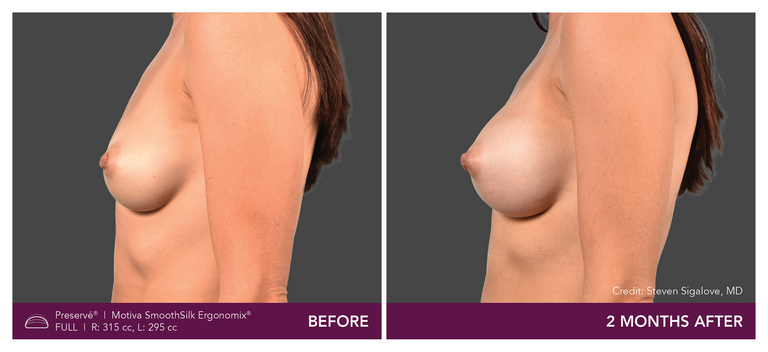
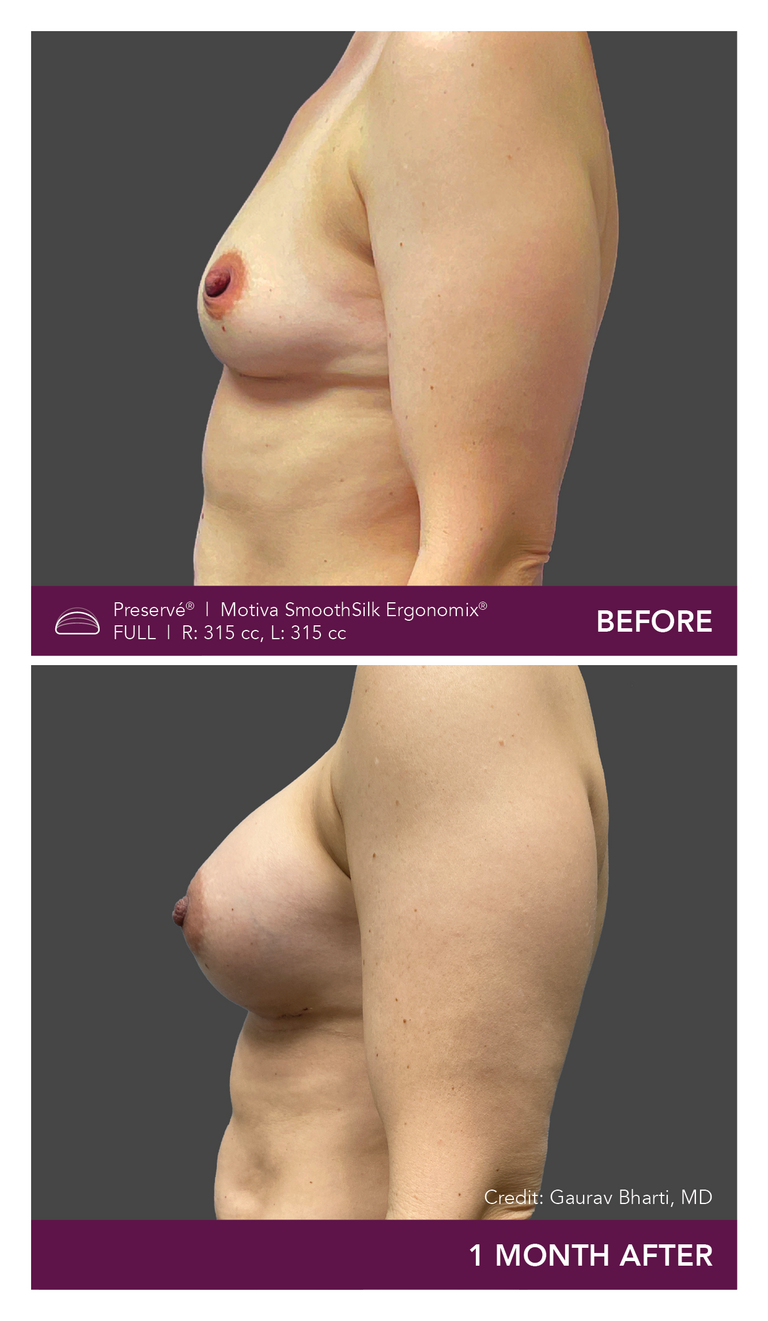
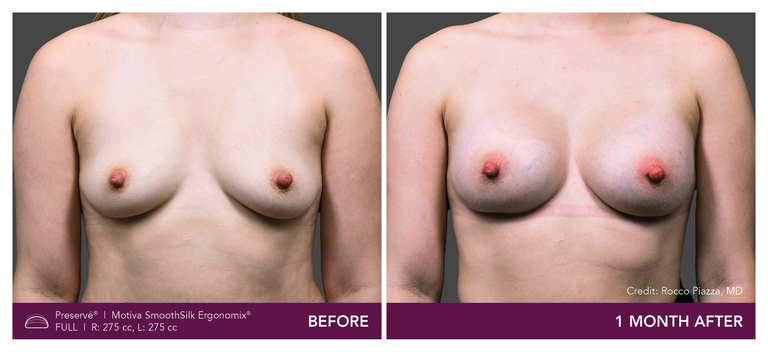
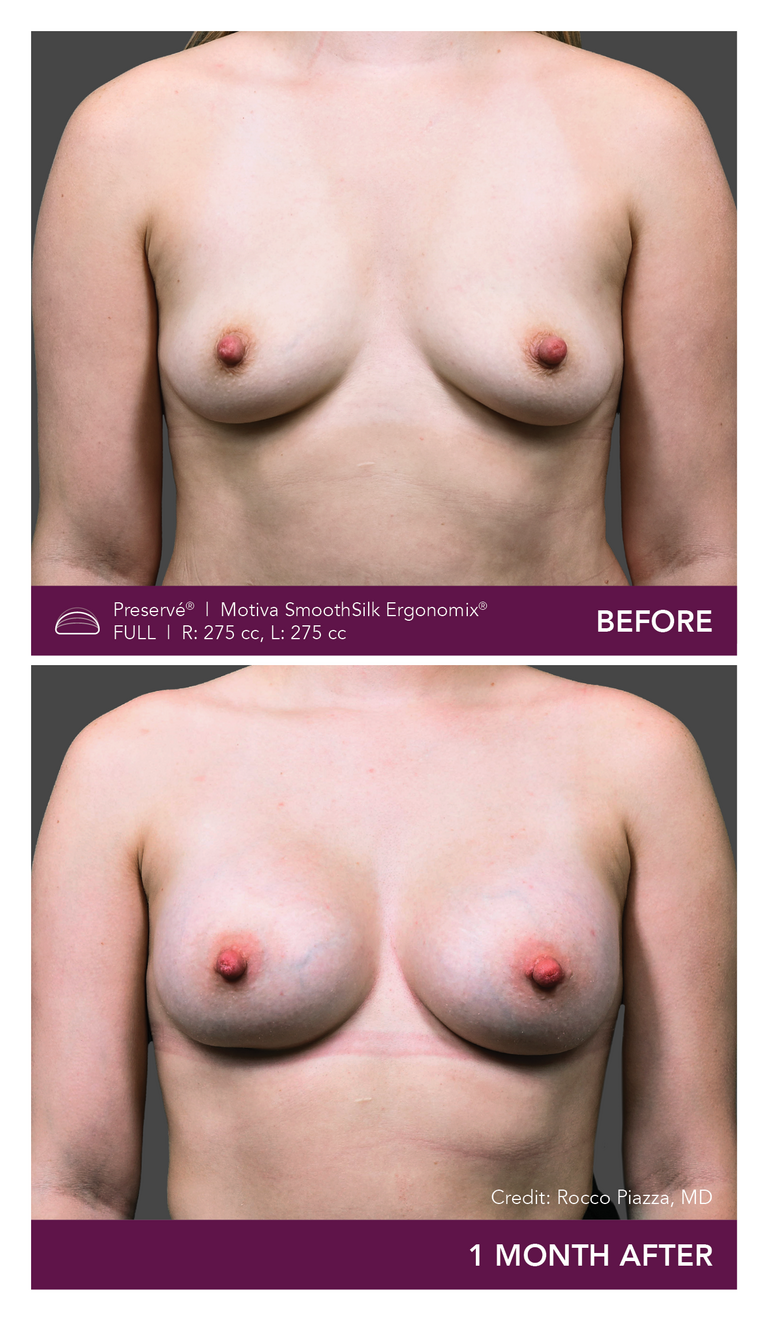
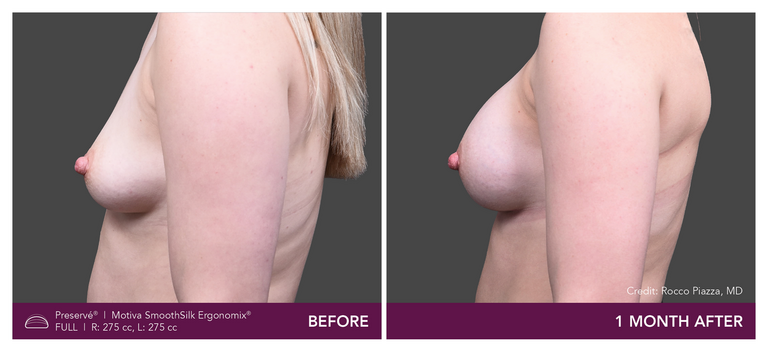
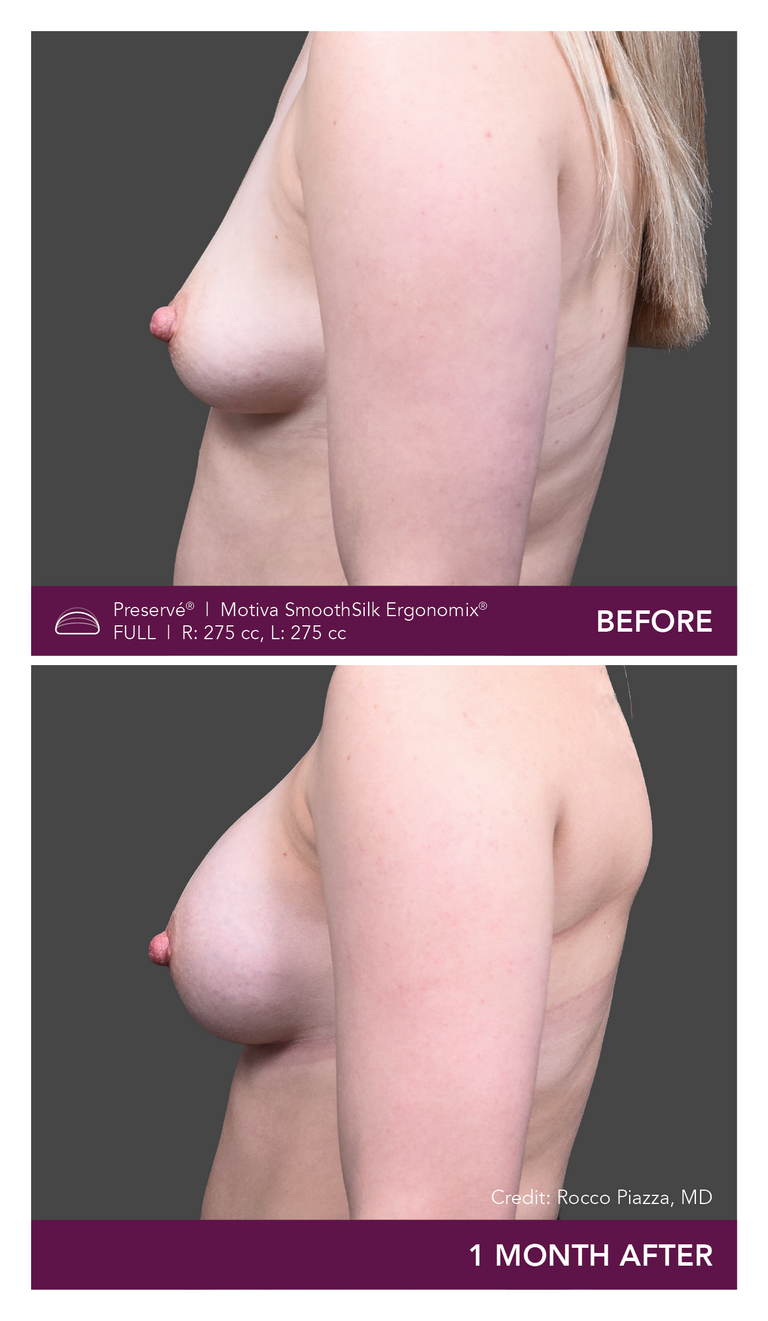
Preservé Before & Afters
Explore patient results by projection.
References
1. Chacon-Quiros M, Sforza M, Solis-Chaves P, et al. The 3-year results of a 100-patient prospective study of safety and effectiveness of Mia Femtech. Aesthet Surg J. 2025;sjaf196. doi:10.1093/asj/sjaf196
2.Establishment Labs, CLIN-001037: Motiva® Breast Tissue Preservation™ Techniques. Data on File.
3.Tebbets J. Redifining the patient and surgeon experience. 2010. Mosby, Elsevier. ISBN: 978-0-323-04112-6
4.Aitzetmuller-Kleitz ML, Yang S, Wiebinghaus P, Wellenbrock S, Ozturk M, Kuckelhaus M et al. Complication rates after breast surgery with the Motiva Smooth SilkSurface silicone gel implants - A systematic review and meta-analysis. Clin. Med. 2023, 12,1881. doi: 10.3390/jcm12051881
5.Establishment Labs, TR-001038: Rheological analysis of silicone filling gels of Motiva Implants® and other brands’ silicone filling gels using the BTC-2000. Data on File.
6.Establishment Labs, DDD-002: Device Description Document for Sterile Silicone Breast Implants Motiva Implants® Ergonomix® Round SmoothSilk®/SilkSurface®. Data on File.
7.Zeplin PH. Narbensparende Brustvergrößerung: Erfahrungen mit über 500 Implantaten. Handchirurgie · Mikrochirurgie · Plastische Chirurgie. 2021;53(02):144-148. doi:10.1055/a-1307-3917
8.Doloff JC, Veiseh O, de Mezerville R, et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nature Biomedical Engineering. 2021. doi:10.1038/s41551-021-00739-4
9. Establishment Labs, DES-001042: Channel Separator Instructions for Use. Internal Data on File.
10. Establishment Labs. CLIN-001033: Clinical assessment of the Motiva®Channel Separator intended use update. Data on file
11. Establishment Labs. TD-001004 Motiva inflatable balloon technical document. Data on file.
12. Establishment Labs. DOC-001009 Motiva Inflatable balloon directions for use. Data on file.
13. Glicksman C, Wolfe A, McGuire P. The Study of the Safety and Effectiveness of Motiva SmoothSilk Silicone Gel-filled Breast Implants in Patients Undergoing Primary and Revisional Breast Augmentation: 5-Year Clinical Data. Aesthet Surg J. 2025 Nov 20:sjaf245. doi: 10.1093/asj/sjaf245. Epub ahead of print. PMID: 41266083.
Preservé® clinical outcome data is currently based on the use of the Motiva Channel Seperator, the Motiva Inflatable Balloon and the Ergonomix2 implant in minimally invasive procedures in Motiva’s OUS markets. While the Ergonomix2 implant is not approved in the U.S., the Motiva Inflatable Balloon and the Motiva Channel Seperator used for the Breast Tissue Preservation System are registered with the FDA and may be used with FDA approved implants
The sale and distribution of this device are restricted to users and/or user facilities that provide information to patients about the risks and benefits of this device in the form and manner specified in the approved labeling provided by Motiva USA.
IMPORTANT SAFETY INFORMATION
The Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants are indicated for breast augmentation for women of at least 22 years old. Breast augmentation includes primary breast surgery to increase the breast size, as well as revision surgery to correct or improve the result of an original primary breast augmentation surgery (i.e., revision-augmentation). Breast Implant surgery is contraindicated in women with active infection anywhere in their bodies, with existing cancer or pre-cancer of their breast who have not received sufficient treatment for those conditions, or who are currently pregnant or nursing. Adequate studies have not been performed to confirm the safety of breast implant surgery in women with these conditions or under these circumstances; therefore, if you have any of the conditions or circumstances listed above, breast augmentation surgery with implants should not be performed at this time. Failure to take into consideration these contraindications may increase the risks involved with breast implant surgery and have the potential to cause harm. Patients should be advised that key complications have historically been associated with silicone gel breast surgery and implantation of silicone gel breast implants including, but not limited to, capsular contracture, implant removal, reoperation, infection, and rupture. Further, breast implants are not lifetime devices and patients should visit their healthcare professional, as recommended. For more detailed information about the benefits and risks of Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants, please visit: www.motivausa.com or call Motiva at 1-800-924-5072.
Motiva manufactures the Preserve® system to support surgeons conducting a less invasive procedure for breast augmentation. Please consult your local surgeon to determine if the procedure described in this advertisement is right for you. Techniques and treatment may vary based on patient circumstances and will ultimately be determined by the treating surgeon.
Motiva®, Establishment Labs®, Aesthetic BreastRecon®, and Ergonomix® are trademarks of Establishment Labs Holdings® Inc.